-
Dexiang: Delivering Trust. Your Reliable Environmental Test Chamber Manufacturer.
- WhatsApp:+8615112812380
What Are Stability Chambers and Why Do They Matter
Stability chambers are controlled environment units designed to test how products respond to varying temperature, humidity, and other conditions over time. These chambers are essential tools for pharmaceutical, food, cosmetic, and electronic industries to ensure product quality and shelf life.
Role in ICH and FDA Compliance
Stability chambers help you meet critical regulatory standards including ICH (International Council for Harmonisation) guidelines and FDA requirements. Using these chambers, you can perform stability testing to prove that your products remain safe and effective throughout their intended shelf life.
Benefits of Stability Testing
- Ensures product reliability under different environmental stresses
- Identifies degradation products early to prevent safety issues
- Supports regulatory submissions with validated data
- Reduces recalls by confirming packaging and formulation stability
Common Parameters Controlled In Stability Chambers
| Parameter | Typical Range | Purpose |
|---|---|---|
| Temperature | 5°C to 60°C | Simulates storage and transit environments |
| Relative Humidity | 20% to 95% RH | Tests moisture impact on product stability |
| Light Exposure | Specific wavelengths/intensity | Evaluates photostability, especially for light-sensitive products |
| Airflow | Controlled circulation | Promotes uniform conditions inside chamber |
Understanding these basics helps you appreciate why stability chambers are vital to product development and regulatory compliance. They provide confident, reliable data that keeps your products safe, effective, and ready for market.
How Many Types of Stability Chambers Exist Breaking Down the Classifications
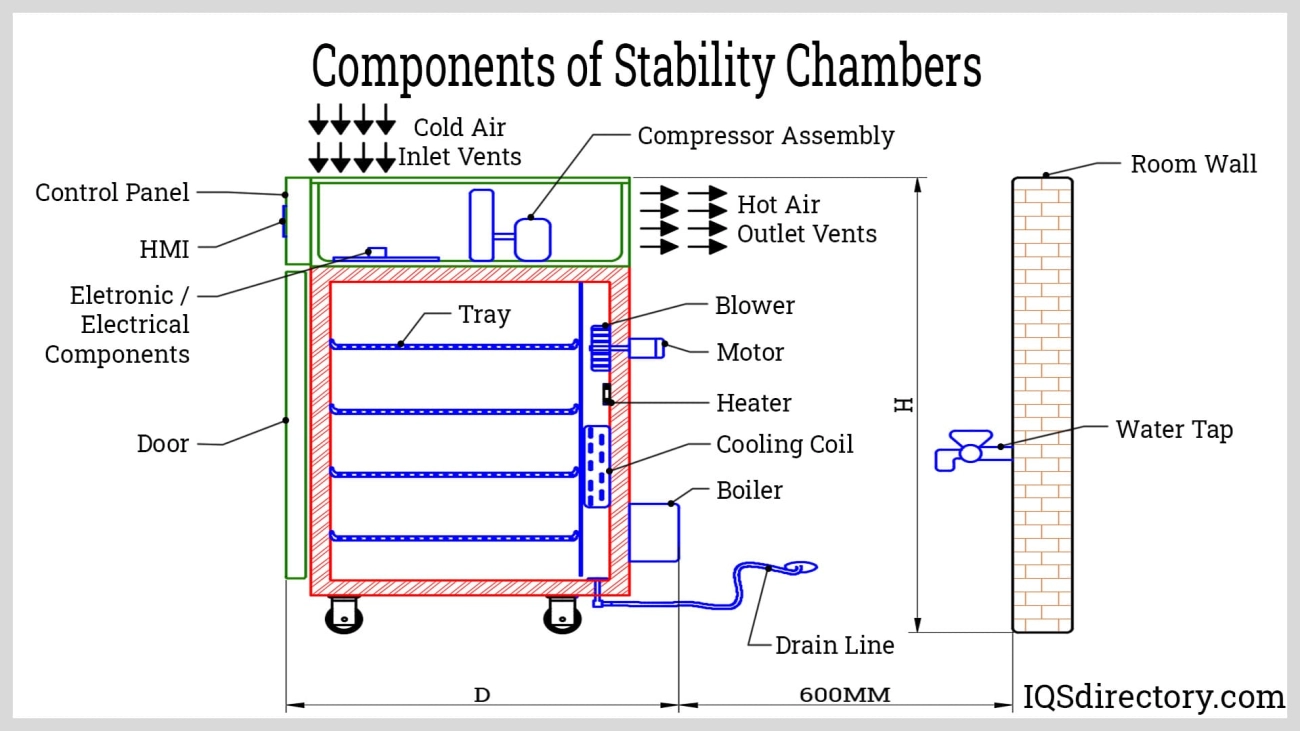
There isn’t a one-size-fits-all number when it comes to types of stability chambers, but they generally fall into several common categories. Each type serves different testing needs based on size, environment control, and specific industry demands.
Here’s a quick breakdown:
| Chamber Type | Size/Capacity | Main Application |
|---|---|---|
| Reach-In Stability Chambers | Compact, small volume | R&D, limited sample testing |
| Walk-In Stability Chambers | Large scale, bulk | Pharma batch testing |
| Photostability Chambers | Varies | Light exposure testing per ICH Q1B |
| Humidity-Controlled Chambers | Medium to large | Precise relative humidity control |
| Thermal Shock and Accelerated Chambers | Small to medium | Rapid stress and shelf-life data |
| Environmental and Climate Chambers | Varies | Multi-parameter control for many industries |
| Custom and Specialized Chambers | Niche sizes | Vacuum, cryogenic, or unique environments |
These classifications help you pick a chamber that fits your testing scope — whether that’s pharmaceutical stability testing, food packaging reliability, or electronics durability. For customers in the U.S., choosing the right type means considering sample volume, regulatory needs (like FDA and ICH compliance), and the specific environmental stresses your products face.
If you need a compact chamber for precise control in drug formulation labs, a Reach-In Chamber works best. For large-scale stability runs, Walk-In Chambers handle bigger volumes efficiently. And if your testing requires light exposure, Photostability Chambers are a must.
No matter your focus, knowing the differences between these types helps you match your testing setup perfectly.
Key Features to Look for in Stability Chambers
When choosing stability chambers, focus on these essentials to get reliable, efficient performance:
Precision Controls and TechnologiesAccurate temperature and humidity control is a must for ICH stability testing.
Advanced sensors ensure consistent conditions for drug shelf life testing equipment.
Digital controllers with real-time data logging help monitor test progress easily.Safety Features and Compliance ValidationChambers should meet FDA and GMP compliant stability room standards.
Built-in alarms for temperature or humidity deviation protect your samples.
Validation tools like mapping and calibration support regulatory compliance.Energy Efficiency ConsiderationsLook for energy-saving designs to reduce operating costs.
Eco-friendly refrigerants and insulated chambers help cut power use.
Smart controls can optimize chamber performance without wasting energy.User-Friendly Interfaces and Monitoring
| Feature | Benefit |
|———————-|————————————————|
| Touchscreen Control | Simple setup and adjustments |
| Remote Monitoring | Check conditions anytime via mobile or desktop |
| Data Export Options | Easy reporting for audits and compliance |
Choosing a stability chamber with these features supports smooth, accurate testing while keeping costs and hassle low.
Applications Across Industries
Stability chambers play a crucial role in many industries, helping businesses ensure their products hold up over time under specific environmental conditions.
- Pharmaceuticals: These chambers are essential for drug shelf-life testing and API (active pharmaceutical ingredient) stability. They help meet FDA and ICH guidelines by simulating real-world conditions to make sure medicines remain safe and effective until their expiration date.
- Food and Cosmetics: Stability testing in these sectors focuses on packaging durability and preventing microbial growth. Chambers simulate temperature and humidity to verify how well products maintain quality and safety on the shelf.
- Electronics and Biotechnology: Here, stability chambers test component reliability under different temperature and humidity conditions to reduce failure risk during use. This is key to ensuring long-term performance and safety.
- Dexiang Support for Tropical Zone Testing: We provide specialized stability chambers designed for hot and humid environments like tropical zones. This helps U.S. businesses and exporters prepare their products for global markets with challenging climates.
By customizing stability chambers to fit specific industry needs, Dexiang helps companies in the U.S. stay compliant, improve product quality, and reduce risk across the board.
ICH Guidelines and Regulatory Compliance
When it comes to stability chambers, following ICH guidelines is critical—especially Q1A to Q1F. These guidelines lay out the necessary conditions for stability testing, covering everything from temperature and humidity settings to storage durations. They help ensure your product maintains quality, safety, and efficacy throughout its shelf life.
Validation is a must. This means you’ll need thorough mapping and calibration of your chamber to prove it can consistently hold precise conditions. Mapping checks temperature and humidity uniformity across the chamber, while calibration verifies that sensors report accurate data. Both steps are essential for FDA and GMP compliance.
If you’re dealing with multiple regulatory zones—like tropical, subtropical, or dry climates—choose chambers that can be programmed for those specific conditions or have multi-zone control. This simplifies compliance with global standards and helps avoid costly delays or product recalls.
In short:
- Follow ICH Q1A to Q1F for environmental conditions and testing protocols
- Validate your chamber through mapping and sensor calibration
- Pick chambers with multi-zone or customizable settings for global regulatory needs
This approach keeps you compliant, protects your products, and supports smooth regulatory approvals across the board.
Choosing the Right Stability Chamber A Buyers Guide
Picking the right stability chamber depends on a few key factors:
- Sample volume: How much product do you need to test? Smaller batches might do well with reach-in chambers, while large-scale pharma testing usually requires walk-in models.
- Budget: Balance what you need with what you can afford. More features and custom options usually mean a higher price.
- Testing needs: Consider what parameters you must control—temperature, humidity, light exposure—and whether you need accelerated or long-term stability testing.
Common mistakes to avoid include:
- Overlooking uniformity and consistency inside the chamber, which can lead to unreliable results.
- Neglecting routine maintenance and calibration, which impacts accuracy and lifespan.
At Dexiang, we offer customized stability chamber solutions tailored to your exact requirements. Plus, our after-sales support ensures your equipment runs smoothly long after purchase, minimizing downtime and maximizing compliance. We understand the unique demands of U.S. industries and help you meet FDA and ICH standards without hassle.
Maintenance Calibration and Best Practices
Keeping your stability chambers in top shape is crucial for reliable results and long equipment life. Here are some key maintenance and calibration tips that help you get the most from your investment.
Routine Maintenance Tasks and Checks
- Clean chamber interiors regularly to prevent contamination and buildup.
- Inspect door seals and gaskets for any damage or leaks that can affect temperature and humidity control.
- Check and replace filters as needed to maintain airflow and clean operation.
- Monitor sensors and probes for accuracy and replace if they show signs of wear.
- Lubricate moving parts like hinges and fans to avoid mechanical issues.
- Review alarm systems to ensure they activate properly during deviations.
Troubleshooting Common Issues
- Uneven temperature or humidity may indicate sensor problems or airflow blockages.
- Frequent power outages or chamber resets suggest electrical faults or overload.
- Condensation inside the chamber can point to sealing problems or high humidity settings.
- Unusual noises often come from faulty fans or worn mechanical parts.
- Always refer to the manufacturer’s manual for step-by-step troubleshooting.
Extending Chamber Lifespan with Professional Services
- Schedule annual professional calibrations to keep sensors and controls accurate and compliant with ICH and FDA regulations.
- Use certified service technicians for repairs and upgrades to maintain GMP standards.
- Keep a maintenance log to track issues, repairs, and performance over time.
- Consider custom service plans for your specific environment and testing needs.
Regular care and precise calibration not only ensure your stability chambers perform well but also protect your product data integrity and regulatory compliance.
FAQs on Types of Stability Chambers
How many types of stability chambers are standard in the pharmaceutical industry?
While there’s no one fixed number, most pharmaceutical labs rely on about 5 common types: reach-in, walk-in, photostability, humidity-controlled, and accelerated or thermal shock chambers. These cover the main stability testing needs outlined by ICH and FDA guidelines.
What’s the difference between reach-in and walk-in stability chambers?
Reach-in chambers are smaller, ideal for research, development, or limited samples. Walk-in chambers are much larger, designed for bulk testing of pharmaceutical batches or products requiring more space and consistent environmental control.
Are photostability chambers always required in testing?
Yes, photostability chambers are essential for testing how products react to light exposure. This is a critical part of ICH Q1B guidelines to ensure drug stability under light and helps meet regulatory compliance.
What should buyers know when choosing stability chambers?Consider the volume of your samples.
Match chamber capabilities with your testing protocols (temperature, humidity, light).
Look for compliance with GMP and FDA standards.
Energy efficiency and user-friendly controls save costs and time.
Don’t overlook maintenance support and calibration services.Can stability chambers handle varied testing conditions?
Many modern chambers come with multi-parameter control for temperature, humidity, and light — allowing versatile testing across industries like pharmaceuticals, food, cosmetics, and electronics.
Ready to enhance your product testing with a reliable, precision-built solution? As your trusted environmental test chamber manufacturer, we invite you to contact Dexiang for a free brochure or to schedule a live demo.
Dexiang| Instruments
As a high-tech manufacturer, we integrate R&D, design, production, and sales under one roof. Our philosophy—"Quality Ensures Survival, Integrity Drives Development, and Management Yields Efficiency"—is woven into every product we build. We continuously assimilate cutting-edge global technologies and refine our practices through years of hands-on experience.
相关推荐
- How to Choose the Right Environmental Test Chamber Manufacturer 2026
- How to Choose Between IPX34 Rain Test and Dust Test Chambers
- Salt Spray vs Cyclic Corrosion Chambers Differences and Buying Guide
- Testing Standards for Salt Spray Test Chambers | ASTM B117 and ISO 9227 Guide
- Top Xenon Lamp Aging Test Chamber Standards Buyers Must Know 2026
Dexiang Environmental test chamber manufacturer All Rights Reserved.



